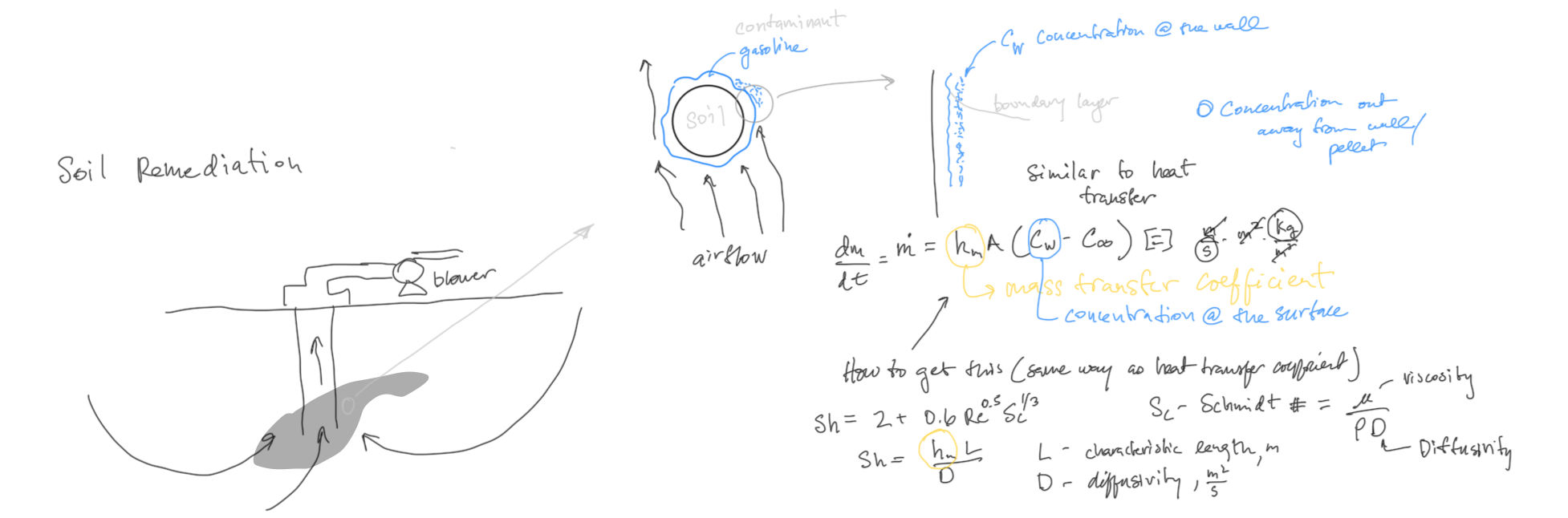
In previous discussions on environmental remediation, we have discussed ways in which material can be transferred from one location to another or that it can be broken down and then transferred. The rate of that transfer depends on the concentration immediately next to the surface and out in the middle of the fluid. Principles of vapor-liquid equilibrium (concentration next to the surface) and mass transfer are reviewed here.
Heat and Mass Transfer Preview¶
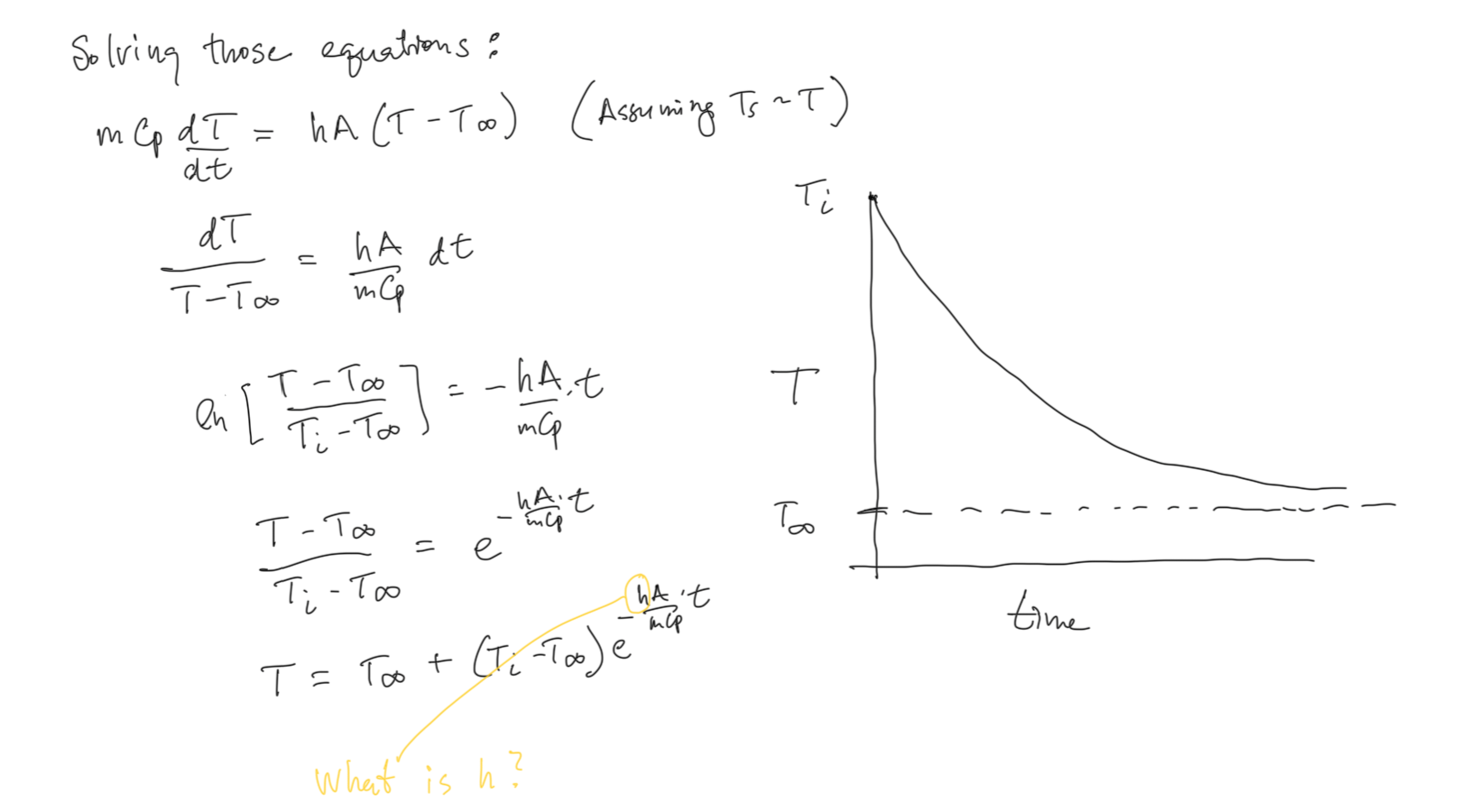
Heat Transfer: Convection¶


Mass Transfer: Convection¶

Vapor Liquid Equilibrium Preview¶
Raoult’s Law¶
Henry’s Law¶
Henry’s Law states that the concentration of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. The solubility of a gaseous species is dependent on the temperature and pressure of the system. Henry’s Law can be used to predict the concentration of a species in a liquid:
where: is the mole fraction of species i in the gas phase, is the pressure of the gas above the liquid, is the Henry’s Law constant for the species i, and is the mole fraction of the species in the liquid.
Henry’s law is very similar to Raoult’s Law, which states that the partial pressure of a species in the vapor phase is directly proportional to the product of the mole fraction of the species in the solution and the pure component saturated vapor pressure of that species. Both ‘laws’ are limit cases where Henry’s Law is for dilute solutions and Raoult’s Law is for ideal solutions.
- Guymon, C. (2025). Foundations of Spiritual and Physical Safety: with Chemical Processes.